Article In Press
Chemical and Functional Properties of Sorghum-Acha Cereal Blends Enriched with Cricket Whole Meal, Defatted Protein Meal, And Protein Hydrolysate
Anih Peace Ogomegbunam*, Girgih Abraham Tartenger, Ikya Julius Kwagh-al
Department of Food Science and Technology, Joseph Sarwuan Tarka University, Makurdi, Nigeria
*Corresponding author: Anih Peace Ogomegbunam, Department of Food Science and Technology, Joseph Sarwuan Tarka University, Makurdi, Nigeria, Tel: +2348032936169 & 2348029383858, E-mail: [email protected]
Received Date: May 29, 2025
Publication Date: June 24, 2025
Citation: Ogomegbunam AP, et al. (2025). Chemical and Functional Properties of Sorghum-Acha Cereal Blends Enriched with Cricket Whole Meal, Defatted Protein Meal, And Protein Hydrolysate. Nutraceutical Res. 4(1):12.
Copyright: Ogomegbunam AP, et al. © (2025).
ABSTRACT
Background: Bioactive compounds are non-nutritive food components that exert significant physiological effects in humans. There are present in small quantities in food but offer potent health benefits, including antioxidant, anti-inflammatory, and anti-carcinogenic properties. With increasing scientific interest, bioactive compounds are being explored for functional food development due to their potential to manage chronic diseases Whole crickets were processed into whole cricket meal (WCM), defatted cricket meal (DCM), and cricket protein hydrolysate (CPH). Each derivative was added at 10% to a base mix of 80% sorghum and 10% acha flour to produce three enriched samples: SAWCM, SADCM, and SACPH. The 100 % (SOF) served as control. Objective: The broad objective of this study was the evaluation of the chemical and functional properties of sorghum-acha cereal blends enriched with cricket whole meal, defatted protein meal, and protein hydrolysate. Methods: All the samples were anallyses using standard analytical methods, the proximate, mineral, vitamin determing using (AOAC, 2012) while Results: Proximate analysis revealed that cricket-enriched blends had significantly higher protein (up to 70%), ash (11%), and fiber (11.5%) contents, with a lower value in fat (3%) and carbohydrate (14%) levels compared to the control. Amino acid profiling showed substantial improvements across all groups as compared to the control. Mineral analysis indicated that the enriched samples were superior sources of both macro and trace minerals. For example, magnesium, calcium, and phosphorus were notably higher, while trace elements like iron (50% increase), zinc (29.8%), manganese (38%), and copper (105%) (p<0.05) differed significantly. Functional properties revealed, cricket extended samples showed superior properties in comparison to the control sample. Conclusion: Findings highlight the potential of cricket-enriched sorghum-acha blends as nutritionally food products, capable of addressing malnutrition and micronutrient deficiencies.
Keywords: Cricket Protein Hydrolysate, Sorghum-Acha Cereal Blend, Bioactive Compounds, Nutritional Enrichment
INTRODUCTION
Bioactive compounds are non-nutritive food components that exert significant physiological effects in humans. These compounds, which include flavonoids, polyphenols, carotenoids, tannins, plant sterols, and glycosylates, are present in small quantities in food but offer potent health benefits, including antioxidant, anti-inflammatory, and anti-carcinogenic properties [1,2]. With increasing scientific interest, bioactive compounds are being explored for functional food development due to their potential to prevent or manage chronic diseases [3].
Interestingly, insects such as crickets have recently gained attention as unconventional but rich sources of bioactive compounds and antimicrobial peptides (AMPs), with evidence showing their potential in supporting immune function and reducing disease risk [4]. Crickets are not only rich in high-quality protein (about 41%) and fat (38%) but also contain essential micronutrients like iron, calcium, and amino acids such as tryptophan [5,6]. They are now widely processed into various food forms such as whole flour, defatted meal, and protein hydrolysates. These forms are used to fortify low-lysine and low-tryptophan cereal-based foods to improve protein quality [7]. Cricket protein hydrolysates, derived through enzymatic cleavage, are particularly valued for their rapid digestibility and enhanced amino acid bioavailability, making them ideal for improving muscle health and overall nutrition [8]. Additionally, due to their high fat content, crickets are sometimes defatted to create more functional and adaptable food products [9]. Sorghum (Sorghum bicolor), the fifth most important cereal globally, is a staple in Nigeria and is valued for its high carbohydrate content and energy provision [10]. However, its protein content (7–10%) lacks essential amino acids such as lysine and tryptophan [11]. This limitation can be addressed by supplementing sorghum-based products with cricket derivatives. Similarly, fonio (Digitaria exilis), ancient cereal rich in methionine, leucine, and valine, is easy to digest and suitable for diabetic, gluten-intolerant, or recovering individuals. It has significant promise as a complementary food ingredient [12]. Despite its superior methionine content—reportedly higher than egg protein—fonio remains underutilized [13]. Both cereal grains and crickets contain some anti-nutritional factors like phytates, tannins, oxalates, and phenols, which may hinder nutrient bioavailability. However, these can be significantly reduced through common food processing techniques [14]. A blend of sorghum and fonio fortified with cricket-based ingredients such as whole flour, defatted flour, and protein hydrolysate could provide a cost-effective, nutrient-dense food product with enhanced bioactive and functional properties. Carrot flour, known for its beta-carotene content, may further boost the health profile of the formulation. Malnutrition, particularly protein-energy malnutrition, remains a critical health issue in Nigeria due to the unaffordability and inaccessibility of conventional animal proteins like meat and fish. Cereal-based foods, widely consumed by both adults and children, often fall short of meeting complete nutritional needs, especially in terms of essential amino acids. Crickets, as a cheap and abundant source of protein and micronutrients, offer a promising solution to bridge this gap through food fortification. Chronic diseases such as diabetes, cardiovascular ailments, and cancer are on the rise globally, and bioactive-rich functional foods are gaining importance in dietary interventions. Developing low-cost, nutrient-rich cereal blends fortified with cricket products aligns with the growing demand for convenient, ready-to-eat foods. Such products could help combat malnutrition, improve public health, and transform agricultural byproducts into valuable nutritional resources. The broad objective of this study was the evaluation of the chemical and functional properties of sorghum-acha cereal blends enriched with cricket whole meal, defatted protein meal, and protein hydrolysate.
MATERIALS AND METHODS
Source of materials
Sorghum and Acha grains were purchased from Modern Market, Makurdi, Benue State. Life edible crickets were purchased from Agasha, Gboko road Benue State. Wistar albino rats weighing (100 -130 g) were obtained from National Veterinary Research Institute, Vom, Jos, Plateau State, Nigeria. Pepsin (porcine gastric mucosa), Pancreatin (porcine pancreas), 2,2-diphenyl-1-picrylhydrazyl (DPPH), Triton X-100, hydrogen peroxide, ethylenediaminetetraacetic acid (EDTA), ferrous sulphate, potassium ferricyanide, trichloroacetic acid (TCA), ferrous chloride, 1,10-phenanthroline, 3-(2-pyridyl)-5,6-diphenyl-1,2,4-triazine-4′,4′′-disulphide acid sodium salt (ferrozine) and GSH were purchased from Sigma-Aldrich (St. Louis, MO) while other analytical grade reagents were obtained from Fisher Scientific (Oakville, ON, Canada).
Blend Formulation for the production of sorghum-acha flour blends enriched with different cricket derivatives
Formulation were as follows, 100 % sorghum (SOF) served as control, SAWCM = 80 % Sorghum + 10 % Acha + 10 %Whole cricket meal, SADCM = 80 % Sorghum +10 % Acha + Defatted cricket meal, SACPH = 80 % Sorghum + 10 % Acha + 10 % Cricket protein hydrolysate.
Preparation of malted sorghum flour
The method described by Marston K, et al. [15] was used with slight modifications for the production of sorghum flour. Sorghum seeds were gotten, sorted, soaked, drained, allowed to germinate, sun dried for 48 hours, milled, sieved using 0.5µm mesh size to obtained malted sorghum flour. The grains (5kg) were sorted and cleaned to remove foreign materials, before washing with tap water and steeping (48 h). Thereafter, the grains were drained, germinated (72 h), dried, milled and sieved to get malted sorghum flour.
Preparation of acha flour
The method described by Ayo J, et al. [16] was used for the production of Acha flour. Acha seeds was gotten, winnowed, washed, sun dried for 48h, milled and sived using (0.5µm mesh size). The grains (3 kg) were sorted and cleaned to remove unwanted materials stones, pebbles and other foreign seeds, before washing with tap water, drained, dried, milled and sieved to get Acha flour.
Preparation of different cricket derivatives
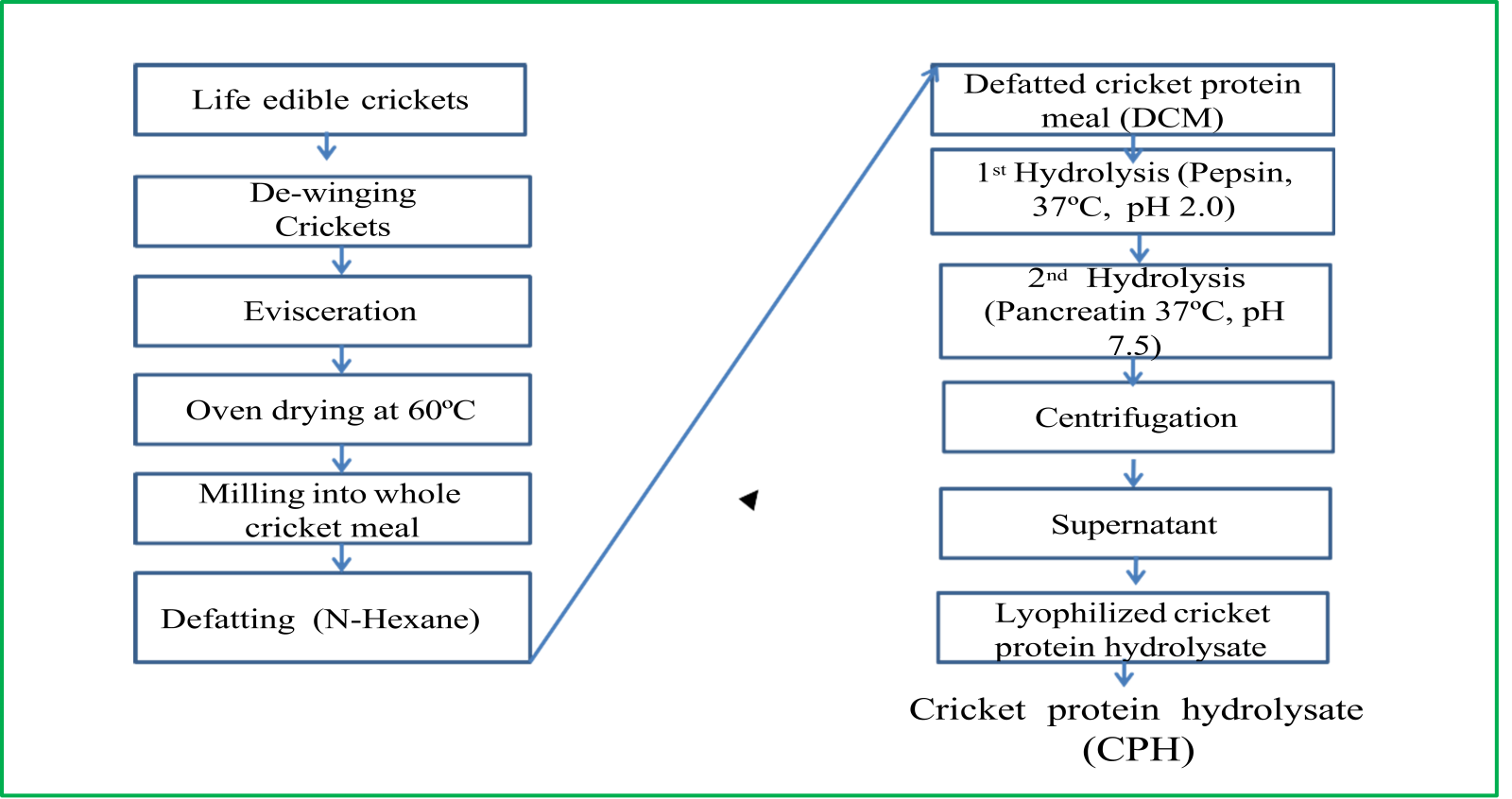
Shortly after the crickets were collected, their wings were removed, eviscerated, washed, oven dried and milled into whole cricket meal. The whole cricket meal was then defatted with n-hexane to remove fat to get defatted cricket. The defatted cricket protein meal was further hydrolyzed with pepsin and pancreatin, centrifuged, the supernatant removed and freeze dried into cricket protein hydrolysate as described in Figure 1.
Figure 1. Preparation of cricket protein hydrolysate.
Source: [17]
Chemical analyses of sorghum-achacricket extended flours
Proximate composition of sorghum-acha cereal blends enriched with cricket whole meal, defatted protein meal, and protein hydrolysate
The ash, moisture, crude fat, crude protein, crude fibre and carbohydrate content of the composite flour were determined according to AOAC [18].
Determination of minerals in sorghum–acha cricket extended flours: The following minerals; sodium (Na), potassium (K), calcium (Ca), iron (Fe), magnesium (Mg) and phosphorous (P) were analyzed according to AOAC [18]. This was determined after drying and ashing in a muffle furnace for several hours until white-grey ash was obtained. When cool, 20 ml of distilled water and 10 ml of dilute hydrochloric acid (HCl) were added to the ashed material. This mixture was boiled, filtered into a 250 ml volumetric flask, washed thoroughly with hot water, cooled and made up to volume. Mineral content of each sample were analyzed using colorimetric or spectrophotometric or titrimetric methods were applicable (AOAC, 2005). Samples were analyzed `for sodium (Na), potassium (K), calcium (Ca), iron (Fe), magnesium (Mg), phosphorus (P), Zinc (Zn), Selenium (Se), Copper (Cu) and Molybdenum (Mo) and estimated quantitatively using an atomic absorption spectrophotometer (scientific model VGP 210) using filters that match the different elements.
Determination of vitamins content of Sorghum–Acha cereal blends enriched with cricket derivatives
Vitamins B1, B2, B3, B6, B9, E were determined using the method described by AOAC [18]. Five (5) g of homogenized sample was weighed into 100 ml volumetric flask. 0.1 N hydrogen chloride was added and mixed then autoclaved for 30 minutes at 121°C. The samples were allowed to cool.
Interfering substances were precipitated by adjusting the pH to 6.0 followed immediately by readjusting the ph to 4.5. This was then diluted to volume with water and filtered. 5 ml of 6 % enzyme (amylase 100) was added and incubated for 3 hours at 45 – 50°C. This was then cooled and H adjusted to 3.5 and diluted with water to volume, mixed and filtered. 10 ml of diluted extract was oxidized by passing through a seppak C18 catridge followed by 5 ml 0.01m phosphate buffer at pH 7.0. The vitamins were separated by high performance liquid chromatography (HPLC) (Model: BLC-10/11, Buck scientific, USA) using a 4.6 mm x 25 cm ultra-sphere ODS (operational data store), 5 column or equivalent and detected by florescence at 360 nm/415 nm(ex/em). The niacin (Vit B3), riboflavin (Vit B2) and thiamin (Vit B1) contents were measured by the calculation below.
Determination of amino acid composition of sorghum–acha cricket extended flours
The amino acid profile of each sample was determined according to the established methods described by Girgih AT, et al. [19] using a HPLC system after hydrolysis with 6 M HCl. The cysteine and methionine contents were determined after performic acid oxidation while the tryptophan content was determined after alkaline hydrolysis.
Determination of the functional properties of sorghum–acha cricket extended flours
Functional Properties was determined follwed the method described by Owuka GI [20] and the pasting properties was analysed using rapid visco analyser.
Statistical analysis
Analyses of the data was performed by comparison using Tukey’s test. Means and Standard deviation were calculated where appropriate. Analysis of variance (One-Way ANOVA) was used to determine the treatment that was different from others in the various parameters tested; differences were considered significant at 95 % (p<0.05) significant level and 99 % (p<0.01) significant level where mentioned.
RESULTS
Proximate composition of Sorghum-Acha cereal blends enriched with cricket derivatives.
Cricket-enriched cereal blends showed significantly higher protein (up to 14.1%), fat, fibre, and ash contents than the sorghum-only sample (SOF). SOF had the lowest moisture (9.10%) and highest carbohydrate (70.3%) content, while SACPH and SAWCM had the highest moisture and fat, respectively. Fibre and ash content peaked in SADCM and SACPH, respectively, with notable differences across all samples (p<0.05). Overall, cricket inclusion improved the nutritional quality of the cereal blends.
Table 1. Proximate composition of Sorghum-Acha cereal blends enriched with cricket derivatives
|
Sample |
Protein (%) |
Ash (%) |
Fibre (%) |
Moisture (%) |
Fat (%) |
Carbohydrate (%) |
|
SOF |
8.31d±0.05 |
3.41c±0.09 |
5.83c±0.44 |
9.10d±0.94 |
3.09c±0.06 |
70.3a ±0.12 |
|
SAWCM |
13.1c±0.95 |
3.78b±0.55 |
6.41a±0.94 |
11.2b±1.03 |
5.02a±0.72 |
60.4c ±0.22 |
|
SADCM |
13.4b±0.55 |
3.78b±0.33 |
6.50a±0.94 |
10.2c±1.04 |
2.99d±0.08 |
63.2b ±0.32 |
|
SACPH |
14.1a±0.99 |
3.87a±0.05 |
6.07b±1.04 |
11.3a±2.04 |
3.96b±1.03 |
60.8c ±0.12 |
Values are means ± standard deviation of duplicate determinations. Means with same superscript in the same column are not significantly (p<0.05) different. Key: SOF =100 % sorghum; SAWCM = 80 % sorghum + 10 % acha +10 % whole cricket meal; SADCM = 80 % sorghum + 10 % acha + 10 % defatted cricket meal; SACPH = 80 % sorghum + 10 % acha + 10 % cricket protein hydrolysate.
Mineral composition of sorghum-acha cereal blend enriched with cricket derivatives
Cricket-extended cereal blends had higher levels of both macro- and trace minerals than SOF pap. Macrominerals like Mg, K, Na, Ca, and P varied significantly, with SOF consistently showing the lowest values. Trace minerals (Cu, Mn, Fe, Zn, Se) also followed this trend, with highest values in blends like SACPH, SAWCM, and SADCM. Statistically significant differences (p<0.05) were observed, confirming improved mineral content in cricket-enhanced samples.
Table 2. Mineral composition of sorghum–acha cereal blend enriched with different cricket derivatives
|
Element |
SOF |
SAWCM |
SADCM |
SACPH |
|
Magnesium |
163.6b±0.43 |
178.4a±2.04 |
177.5a±2.04 |
178.3a±2.05 |
|
Potassium |
362.4a±0.99 |
366.3b±4.03 |
366.1c±5.09 |
368.0d±4.04 |
|
Sodium |
6.35c±1.04 |
7.03b±0.99 |
7.01b±0.55 |
7.08c±0.95 |
|
Calcium |
11.3b±1.05 |
15.4a±1.04 |
15.3a±1.04 |
15.4a±0.99 |
|
Phosphorous |
301.4b±1.11 |
303.8a±2.94 |
304.0a±2.35 |
303.6a±0.54 |
|
Copper |
0.97c±0.04 |
1.90b±0.05 |
1.99a±0.92 |
1.92b±0.99 |
|
Manganese |
1.02b±0.05 |
1.34a±0.03 |
1.36a±0.92 |
1.41a±0.08 |
|
Iron |
3.14c±0.09 |
6.25a±1.03 |
6.20b±0.44 |
6.23b±0.09 |
|
Zinc |
1.66b±0.85 |
4.35a±1.04 |
4.59a±0.55 |
4.48a±0.55 |
|
Selenium |
0.00a±0.00 |
0.005a±0.00 |
0.01a±0.00 |
0.01a±0.00 |
Values are means ± standard deviation of duplicate determinations; Means with same superscript in the same row are not significantly (p<0.05) different.
Key: SOF = 100 % Sorghum; SAWCM = 80 % Sorghum + 10 % Acha +10 % whole cricket meal; SADCM = 80 % Sorghum + 10 % Acha + 10 % defatted cricket meal; SACPH = 80 % Sorghum + 10 % Acha + 10 % cricket protein hydrolysate.
Vitamin content of the cereal blend enriched with cricket derivatives
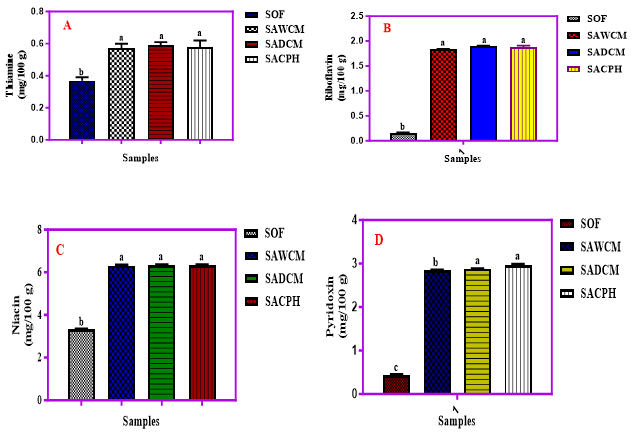
The result of the vitamin content of the cereal blends fortified with cricket products is presented in Figure 2 (A-G). The thiamine content ranged from 0.37 to 0.59 mg/100 g with the defatted cricket sample having the highest value of thiamine. Similarly, the riboflavin content ranged from 0.15 to 1.89 mg/100 g with sample with the defatted cricket meal having the highest value. Niacin content ranged from 3.33 to 6.39 mg/100 g with sample with cricket hydrolysate having the highest value.
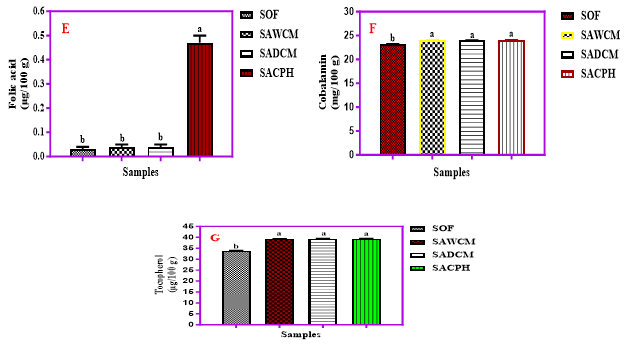
Figure 2 (A-G). Levels of vitamin B1, B2, B3, B6, B9, B12 and E (mg/100g) in Sorghum-Acha flour blends enriched with cricket products, WCM, DCM and CPH; Key: SOF = 100 % Sorghum; SAWCM = 80 % Sorghum + 10 % Acha + 10 % Whole Cricket Meal, SADCM = 80 % Sorghum + 10 % Acha + 10 % defatted cricket meal, SACPH = 80 % Sorghum + 10 % Acha + 10 % cricket protein hydrolysate.
Amino acid composition of cereal blends enriched cricket derivatives
Cricket fortification significantly boosted total and essential amino acid content in sorghum-acha blends, with EAA rising from 2.48% (SOF) to 29.2–32.9%. Acidic amino acids like glutamic and aspartic acids were highest, while arginine content increased over 2000%. Defatting and hydrolysis further enhanced individual amino acids such as threonine, methionine, and leucine.
Table 3. Amino acid composition of sorghum-acha cereal blends enriched with different cricket derivatives (g/100 g)
|
Amino acid |
SOF |
SAWCM |
SADCM |
SACPH |
|
ALA |
0.38c±0.00 |
5.52a±0.01 |
5.50ab±0.01 |
5.49b±0.00 |
|
ARG |
0.15b±0.03 |
3.74a±0.07 |
3.73a±0.05 |
3.75a±0.01 |
|
ASX = Asp + Asn |
0.35b±0.05 |
6.33a±0.05 |
6.33a±0.04 |
6.36a±0.05 |
|
GLX = Glu + Gln |
0.63b±0.04 |
9.05a±0.11 |
9.02a±0.10 |
9.05a±0.02 |
|
GLY |
0.14d±0.04 |
3.74a±0.07 |
3.68b±0.18 |
3.61c±0.04 |
|
HIS |
0.11c±0.02 |
1.92ab±0.09 |
1.89b±0.41 |
1.93a±0.07 |
|
ILE |
0.34d±0.06 |
3.03c±0.04 |
3.09b±0.09 |
3.94a±0.23 |
|
LEU |
0.39±0.05 |
5.38c±0.13 |
5.79b±0.54 |
5.81a±0.01 |
|
LYS |
0.29a±0.02 |
4.82b±0.09 |
4.81b±0.08 |
5.84a±0.04 |
|
MET |
0.28d±0.04 |
1.94c±0.04 |
2.00b±0.06 |
2.40a±0.01 |
|
PHE |
0.11b±0.02 |
2.70a±0.09 |
2.72a±0.08 |
2.73a±0.09 |
|
PRO |
0.26b±0.03 |
4.53a±0.11 |
4.07c±0.11 |
4.28b±0.11 |
|
SER |
0.18c±0.02 |
3.82b±0.09 |
3.96a±0.66 |
3.89a±0.09 |
|
THR |
0.26d±0.05 |
2.92c±0.09 |
2.99b±0.09 |
2.99a±0.15 |
|
VAL |
0.17c±0.03 |
4.52a±0.10 |
4.44b±0.11 |
4.46ab±0.09 |
|
TRP |
0.09c±0.02 |
0.72a±0.02 |
0.60c±0.09 |
0.72a±0.04 |
|
EAA |
2.48 ±0.02 |
29.2±0.11 |
29.6±0.41 |
32.9±0.24 |
|
HAA |
1.92±0.15 |
23.1±0.10 |
23.4±0.33 |
25.2±0.21 |
|
AAA |
0.37±0.35 |
2.66±0.24 |
2.60±0.15 |
3.10±0.22 |
|
SCAA |
0.28±0.25 |
1.94±0.13 |
2.00±0.44 |
2.40±0.07 |
|
PCAA |
0.51±0.12 |
8.66±0.05 |
8.60±0.08 |
9.70±0.05 |
|
NCAA |
0.98±0.03 |
15.2±0.44 |
15.4±0.04 |
15.4±0.09 |
|
BCAA |
0.90±0.35 |
12.9±0.11 |
13.5±0.11 |
14.2±0.09 |
|
TAA |
5.03±0.23 |
77.6±0.25 |
78.1±0.11 |
81.4±0.04 |
GLX= glutamic acid + glutamine; ASX = Aspartic acid + Asparagine; Combined total of hydrophobic amino acids (HAA) = alanine, valine, isoleucine, leucine, tyrosine, phenylalanine, tryptophan, proline, methionine and cysteine; aromatic amino acids (AAA) = phenylalanine, tryptophan and tyrosine; positively charged amino acids (PCAA) = arginine, histidine, lysine; negatively charged amino acids (NCAA) = GLX + ASX, threonine, serine; essential amino acids (EAA) = histidine, isoleucine, leucine, lysine, methionine, phenylalanine, threonine, tryptophan valine and tyrosine; total sulphur-containing amino acids (SCAA) = methionine, cysteine; branched chain amino acids (BCAA)=Leucine, Isoleucine & valine.
Functional properties of cereal blend enriched with cricket derivatives
Cricket-fortified samples showed improved functional properties like higher oil and water absorption, bulk density, and foam capacity compared to sorghum-only (SOF). Defatted cricket meal had the highest water absorption, while whole cricket meal led in oil absorption and foam capacity. SOF had the highest swelling index and peak viscosity, while cricket blends showed higher gelatinization temperatures.
Table 4. Functional properties of Sorghum-Acha flour blend enriched with cricket derivatives
|
Parameter |
SOF |
SAWCM |
SADCM |
SACPH |
|
Bulk density (g/mL) |
0.89c±0.06 |
1.11a±0.05 |
0.99b±0.06 |
0.93c±0.11 |
|
Water absorption capacity (g/g) |
1.93d±0.05 |
2.18b±0.99 |
3.59a±0.99 |
2.10c±0.04 |
|
Oil absorption capacity (g/g) |
1.24b±0.09 |
1.29a±0.44 |
1.14c±0.06 |
1.27ab±0.08 |
|
Foam capacity (%) |
16.2c±1.03 |
21.4a±1.03 |
21.0b±0.05 |
21.2b±0.99 |
|
Swelling index |
6.71a±0.55 |
5.93b±0.94 |
5.94b±0.44 |
5.57c±0.22 |
|
Gel temperature (ºC) |
59.9c±1.94 |
66.5a±1.04 |
66.6a±0.99 |
64.3b±1.93 |
|
Peak viscosity (RVU) |
343.4a±3.05 |
320.1b±4.03 |
316.3d±2.03 |
318.3c±3.93 |
Values are means ± standard deviation of duplicate determinations. Sample means with same superscript letters in the same column are not significantly (p<0.05) different; Key: SOF = 100 % Sorghum; SAWCM = 80 % Sorghum + 10 % Acha + 10 % whole cricket meal; SADCM = 80 % Sorghum + 10 % Acha + 10 % defatted cricket meal; SACPH = 80 % Sorghum + 10 % Acha + 10 % cricket protein hydrolysate.
DISCUSSION
Proximate compositionof sorghum-acha cereal blends enriched with cricket whole meal, defatted protein meal, and protein hydrolysate
The proximate composition of composite flours from sorghum, acha, and cricket flours (Table 4) revealed protein content ranging from 8.13% in sorghum-only samples to 14.1% in those with cricket flour, showing significant (p<0.05) differences. The increase, a 73.4% rise, implies cricket flour significantly enhances protein value. This aligns with [21], who reported a two-fold rise in protein in porridge blends with cricket meal. The implication is the potential of crickets to nutritionally enrich cereal-based products. Bolarinwa IF, et al. [22] similarly found a 6.56% increase in protein via soy enrichment. Duda A, et al. [23] confirmed cricket powder significantly boosts protein in pasta, supporting findings here. Ghosh A, et al. [24] reported insects contain 44.2–58.3% protein, and González CM, et al. [25] documented 45–47%, validating the high contribution from crickets. Ash content ranged from 3.41% to 3.87%, with highest values in cricket-containing samples. Since ash reflects mineral content, this suggests enhanced mineral richness in the cricket blends. Sorghum-only samples had the lowest ash content. These results were higher than 1.76% for malted sorghum [22] and 2.73% for acha [26], indicating cricket inclusion may elevate dietary mineral availability. El Hosry L, et al. [27] notes that higher ash content translates to better mineral composition. Fibre content ranged from 5.83% in sorghum-only flour to 6.50% in blends with cricket products, marking an 11% increase. This suggests cricket flour contributes notable dietary fibre. While Olapade AA & Aworh OC [26] confirmed acha has low fibre (0.40%), Ghosh A, et al. [24] reported 6.26–11.1% fibre in insects, supporting this outcome. The slightly lower fibre (6.1%) in hydrolysate samples compared to whole or defatted cricket flour may be due to enzymatic digestion breaking down insoluble fibre into soluble forms, thereby reducing total fibre content. This fibre increase has positive implications for digestive health, as noted by Rao SSC, et al. [28], who highlighted fibre’s role in managing constipation and IBS. Moisture content ranged from 9.10% to 11.3%, lowest in sorghum-only samples and highest in those with cricket whole flour. Higher moisture could affect shelf life negatively, as foods with more moisture spoil faster. The values, though higher than 6.54% reported for malted sorghum [22] and 7.88% for acha [29], may be attributed to the composition of cricket flour and the hygroscopic nature of hydrolysates. This characteristic makes hydrolysates absorb moisture more readily post freeze-drying, hence increasing the final moisture content. Fat content ranged from 2.99% to 5.02%, with cricket whole flour samples having the highest fat and defatted flour the lowest. The significant (p<0.05) variation indicates that cricket fat content reported between 15.4–40.5% by Ghosh A, et al. [24] contributes substantially to the blend's lipid level. The implication is twofold: increased energy density in the final product and potential enhancement of fat-soluble nutrient absorption. The lower fat in defatted cricket flour confirms effective removal of lipids and suits formulations needing reduced fat.
Mineral composition of sprouted sorghum, acha flours and cricket meal blends
Understanding the role of macro and trace minerals is essential in evaluating the nutritional enhancement offered by cereal-insect flour blends. As stated by Tosic N, et al. [30], macro-elements such as calcium, potassium, and magnesium are vital for cellular processes, while trace minerals like zinc, iron, and selenium play roles in immunity, blood formation, and enzymatic function. The sodium content ranged from 6.34 to 7.08 mg/100 g, with cricket-blended samples generally higher than the control, confirming cricket flour’s sodium contribution as noted by Bessette AP [31]. Although modest, these levels help regulate osmotic balance and plasma volume [32]. Cricket flour addition also elevated calcium levels (11.3–15.4 mg/100 g), consistent with Bessette AP [31] and Ayo J, et al. [16]. While still below the RDA (1000 mg), these blends offer a functional supplement for bone health [33]. Zinc ranged from 1.65 to 4.58 mg/100 g, with cricket blends outperforming the control. Though lower than values reported by Ghosh A, et al. [24], these levels are relevant to immunity and reproductive health [33], supporting their dietary inclusion.
Magnesium levels were highest in cricket-enhanced samples (up to 178.4 mg/100 g), aligning with findings by Ayo J, et al. [16]. Given magnesium’s role in nerve transmission and energy metabolism, these blends offer a meaningful nutritional benefit. Iron content improved significantly with cricket flour (2.10–2.29 mg/100 g), enhancing hemoglobin formation potential, albeit below the levels reported by Ghosh A, et al. [24]. These findings support its use in combating iron deficiency [34]. Other trace minerals like manganese (1.12–1.41 mg/100 g), selenium (up to 0.05 mg/100 g), and copper (up to 1.99 mg/100 g) were also boosted by cricket inclusion. These nutrients support antioxidant defense, reproductive health, and enzymatic activity [35-36]. Potassium and phosphorus contents were moderately elevated, with cricket hydrolysate contributing the highest potassium (368.0 mg/100 g) and phosphorus reaching up to 310.4 mg/100 g. Both minerals are critical for nerve function and energy metabolism [26,24]. In sum, cricket flour integration significantly improves the mineral profile of cereal blends. These fortified products present a promising strategy to combat micronutrient deficiencies in cereal-dependent populations.
Vitamin contents of sorghum-acha cereal blends enriched with cricket whole meal, defatted protein meal, and protein hydrolysate
The thiamine (Vitamin B1) content in the samples ranged from 0.37 to 0.59 mg/100 g, with the sorghum-only sample having the least (0.37 mg/100 g). Cricket-extended blends showed no significant differences (p>0.05) in thiamine levels despite processing. Jomova K, et al. [37] and Abraham SI, et al. [38] reported higher thiamine contents (0.57–0.89 mg/100 g) in ogi blends with maize, millet, and soybean. Chinwe O, et al. [39] reported 0.45 mg/100 g for acha flour. Thiamine supports carbohydrate metabolism, nerve, muscle, and heart function; deficiency may cause fatigue and irritability. Riboflavin (Vitamin B2) content ranged from 0.15 to 1.89 mg/100 g. Cricket-based blends showed no significant differences (p>0.05), suggesting minimal impact from cricket processing. Bessette AP [40] reported riboflavin values between 1.10 and 1.50 mg/100 g in insects. Ibitoye OA, et al. [41] observed thermal degradation of riboflavin during ogi processing. Chinwe O, et al. [39] and Kulamarva AG, et al. [42] reported lower riboflavin in acha and sorghum (0.10–0.13 mg/100 g). Riboflavin is vital for energy metabolism and redox reactions; its deficiency leads to sore throat, angular stomatitis, and skin inflammation [41-52]. Niacin (Vitamin B3) values ranged from 3.33 to 6.33 mg/100 g, with the highest in the cricket protein hydrolysate (CPH) sample. Cricket inclusion nearly doubled niacin levels compared to sorghum alone. Ochanda SO, et al. [44] reported significantly lower values (0.15–0.16 mg/100 g) for sorghum; Chinwe O, et al. [39] reported 1.56 mg/100 g for acha. Banwo K, et al. [46] also found lower niacin values in maize and sorghum ogi. Niacin supports brain function, cholesterol regulation, and energy metabolism [44]; however, high doses (>500 mg/day) can cause adverse effects [45]. Pyridoxine (Vitamin B6) ranged from 0.43 to 2.96 mg/100 g. Sorghum-only samples had the least, while cricket-extended blends had significantly higher values (p<0.05). Processing did not significantly alter content in cricket samples. Ibitoye OA, et al. [41] observed a reduction in pyridoxine upon cooking fermented sorghum. Pyridoxine supports amino acid metabolism and neurological functions and treats anemia, epilepsy, and isoniazid-induced complications. Folic acid (Vitamin B9) content ranged between 0.03 and 0.47 µg/100 g, with the sorghum-only sample being the lowest. Addition of cricket products (especially SACPH) increased folic acid levels by ~1467%. Ochanda SO, et al. [44] found 0.02 mg/100 g in sorghum, while Basheer EO, et al. [45] recorded 1.94–1.97 mg/100 g in processed crickets. Folic acid is essential for DNA synthesis and cell division, particularly important during pregnancy [49,50]. It also prevents DNA changes linked to cancer [39].
Cobalamin (Vitamin B12) content in the blends ranged from 23.2 to 24.0 mg/100 g, with the cricket hydrolysate blend showing the highest. No significant differences (p>0.05) were found between the samples. Basheer EO, et al. [45] found lower B12 levels (0.18–0.32 mg/100 g) in crickets. Cobalamin supports hematopoiesis and neurological health [53]. Due to its trace quantities, effective assessment methods are required [39]. Tocopherol (Vitamin E) levels ranged from 33.9 to 39.7 µg/100 g. Sorghum-only samples had the least (33.9 µg/100 g), while cricket-extended blends showed significantly higher values (p<0.05). No differences were observed among cricket samples. Tocopherol acts as a strong antioxidant that neutralizes free radicals [52]. Alpha-tocopherol, specifically, is vital for fertility and antioxidant protection [39].
Amino acid contents of sorghum-acha cereal blends enriched with cricket whole meal, defatted protein meal, and protein hydrolysate
The total amino acid (TAA) content ranged from 4.11 to 67.2 g/100 g, with the highest in the cricket hydrolysate-enriched sample. Essential amino acids (EAA) followed a similar pattern, ranging from 2.48 to 32.9 g/100 g. Acha and cricket inclusion significantly boosted both TAA and EAA. Ghosh A, et al. [24] reported 39–53 g/100 g amino acids in edible insects. Cricket protein hydrolysate notably increased lysine, leucine, isoleucine, methionine, arginine, phenylalanine, aspartic acid, glutamic acid, and serine due to enzyme hydrolysis releasing otherwise bound amino acids [53]. Samples containing cricket hydrolysate and defatted cricket meal had higher aromatic (AAA) and hydrophobic amino acids (HAA), which are crucial in protein structure, antioxidant activity, and metabolic regulation [54]. Hydrophobic amino acids facilitate peptide absorption through the epithelial layer via transcytosis [55,56].
Additionally, the presence of positively charged amino acids (PCAA) helps neutralize oxidative radicals. Dietary levels of branched-chain amino acids (BCAAs) like leucine, isoleucine, and valine are implicated in insulin resistance and obesity [57], suggesting their moderation in aged diets. The inclusion of cricket derivatives in sorghum-acha blends significantly improved the profile and content of several amino acids. Each vitamin parameter showed biological relevance in metabolism, growth, development, antioxidant defense, and disease prevention. These findings emphasize the nutritional advantages of cricket-enriched cereal blends, especially in enhancing micronutrient intake diversity.
Functional properties of composite made from sorghum, acha and cricket hydrolysate
The foam capacity (FC) of the composite flours ranged between 16.2% and 21.4%. The highest was in SAWCM (21.4%) and the lowest in SACPH (16.2%). The inclusion of cricket products improved FC, possibly due to protein quality and processing methods like hydrolysis, which may release air-trapping amino acids. This aligns with values for millet flour [58] and surpasses those for mushroom-acha blends (16), suggesting cricket-plant blends offer functional potential for foam-reliant food applications. Water absorption capacity (WAC) varied significantly among blends (1.93–3.59 g/g), highest in SADCM. This was likely due to protein modification during defatting, releasing water-affine amino acids. Malomo SA & Aluko RE [59] noted polarity and amino acid profiles influence WAC. Higher WAC implies improved digestibility and protein incorporation, supporting use in dough-based products. This exceeds values for blends like acha-mushroom [16] and acha-okara [28], indicating cricket-enriched blends' applicability in baked goods. Oil absorption capacity (OAC), a flavor retention indicator, ranged from 1.14 to 1.29 g/g. SAWCM had the highest, likely due to pre-existing fat content. Cricket-enriched samples showed lower OAC than some wheat-rind or millet flours [60], but were sufficient for flavor retention. This could benefit baking industries where sensorial properties are crucial. Swelling index values (5.57–6.71) were highest in sorghum-only and lowest in SACPH. Addition of cricket flours affected swelling, likely due to starch-protein interactions. Contrary to [29], the swelling index here was inversely related to WAC, indicating differences in the hydrolysate source (animal vs. plant). High swelling is beneficial in bakery products for volume and texture enhancement. Bulk density (BD) ranged from 0.89 to 1.11 mg/ml, with sorghum-only being the lightest and SAWCM the heaviest. Lower BD is favorable for infant food formulations [58], while higher BD reduces packaging volume. The increase in BD with cricket inclusion suggests a denser flour matrix, which can affect storage and processing requirements. Gelatinization temperature (GT) ranged from 59.9 to 66.6 °C. SACPH had the lowest GT, while SADCM was highest. GT increased with the addition of low-starch, high-protein cricket flours, which slow gelatinization. This highlights the starch-protein balance in determining flour behavior during cooking. Peak viscosity (PV) values were between 316.3 and 343.4 RVU. Sorghum-only had the highest PV, suggesting high starch content. Lower PV in cricket-blended flours indicates reduced swelling and thickening due to increased protein. PV is critical for determining cooking quality and paste usability [61,62]. Lower PV values could impact the cooking time and final consistency of cereal-based foods.
CONCLUSION AND RECOMMENDATION
CONCLUSION
The proximate result revealed enriching sorghum and acha flours with cricket products significantly improved the nutritional profile of the composite flours. Protein content increased by up to 73.4%, confirming crickets as an excellent alternative protein source. Ash and fibre contents also improved, indicating enhanced mineral and dietary fibre levels. Moisture and fat contents varied depending on the form of cricket flour used, influencing shelf life and energy density. The addition of cricket and acha flours significantly enhanced the mineral content—such as calcium, magnesium, iron, zinc, sodium, and manganese—of the blends compared to sorghum alone. Cricket flours particularly contributed to increases in sodium and magnesium. No significant differences were found between various processed cricket forms. The addition of cricket-based products to sorghum-acha blends significantly improved the vitamin profile, particularly niacin, pyridoxine, and folic acid, with folic acid increasing up to 15-fold. However, there were no significant effects on thiamine, riboflavin, or cobalamin levels in the extended samples compared to the control. The amino results revealed, that, the addition of cricket hydrolysate and acha flour to sorghum flour significantly increased the total and essential amino acid content, especially hydrophobic, aromatic, and branched-chain amino acids. These amino acids contribute to structural properties and health benefits such as antioxidant activity and disease modulation. Functionals properties revealed, composite flours made from sorghum, acha, and cricket products showed improved water and oil absorption foaming capacities, and bulk density. Cricket inclusion, especially defatted and hydrolyzed forms, enhanced protein content and affected swelling and gelation characteristics.
RECOMMENDATION
The enrichment of sorghum-acha cereal blends with cricket derivatives significantly improved their nutritional and functional qualities. Protein content increased by up to 73.4%, and essential amino acids, particularly lysine and tryptophan, were enhanced—addressing common deficiencies in cereals. Mineral levels, especially calcium, magnesium, and iron, also improved, boosting the blends' potential in combating micronutrient malnutrition. Notably, folic acid content rose up to 15-fold, alongside increases in antioxidant and antidiabetic properties. Functional attributes such as water and oil absorption, bulk density, and foaming capacity were also enhanced. These improvements highlight the blends' suitability for developing affordable, protein-rich foods for malnourished populations. It is therefore recommended that food industries adopt these blends in formulating functional foods. Public health programs should consider these products in combating malnutrition and chronic diseases. Consumer education on the safety and benefits of insect-based foods is essential for broader acceptance. Further research should focus on sensory optimization and product development for commercial scaling.
ACKNOWLEDGEMENTS
None.
CONFLICTS OF INTEREST
The authors declare that there are no conflicts of interest.
REFERENCES
- Dhalaria R, Verma R, Kumar D, Puri S, Tapwal A, Kumar V, et al. (2020). Bioactive Compounds of Edible Fruits with Their Anti-Aging Properties: A Comprehensive Review to Prolong Human Life. Antioxidants (Basel). 9(11):1123.
- Samtiya M, Aluko RE, Dhewa T, Moreno-Rojas JM. (2021). Potential Health Benefits of Plant Food-Derived Bioactive Components: An Overview. Foods. 10(4):839.
- Reis FS, Martins A, Vasconcelos MH, Morales P, Ferreira IC. (2017). Functional foods based on extracts or compounds derived from mushrooms. Trends in Food Science & Technology. 66:48-62.
- Kaur G, Sarao P, Chhabra N. (2023). Therapeutic use of insects and insect products: Entomotherapy. Indian Journal of Entomology. 85(3):1-10.
- Magara HJ, Niassy S, Ayieko MA, Mukundamago M, Egonyu JP, Tanga CM, et al. (2021). Edible crickets (Orthoptera) around the world: distribution, nutritional value, and other benefits—a review. Front Nutr. 7:537915.
- Owoicho MC, Idoko FA, Elijah AUO. (2022). Energy optimization, proximate composition, minerals content and sensory evaluation of cookies: A comprehensive snack produced from defatted cricket. International Journal of Nutrition and Dietetics. 8:25-33.
- Borges MM, da Costa DV, Trombete FM, Câmara AKFI. (2022). Edible insects as a sustainable alternative to food products: An insight into quality aspects of reformulated bakery and meat products. Curr Opin Food Sci. 46:100864.
- Rodríguez-Rodríguez M, Barroso FG, Fabrikov D, Sánchez-Muros MJ. (2022). In vitro crude protein digestibility of insects: A review. Insects. 13(8):682.
- Hossain MS, Small BC, Hardy R. (2023). Insect lipid in fish nutrition: Recent knowledge and future application in aquaculture. Reviews in Aquaculture. 16(1):121-153.
- Espitia-Hernández P, Chávez González ML, Ascacio-Valdés JA, Dávila-Medina D, Flores-Naveda A, Silva T, et al. (2022). Sorghum (Sorghum bicolor L.) as a potential source of bioactive substances and their biological properties. Crit Rev Food Sci Nutr. 62(8):2269-2280.
- Mistry K, Sardar SD, Alim H, Patel N, Thakur M, Jabbarova D, et al. (2022). Plant-based proteins: Sustainable alternatives. Plant Sci Today. 9(4):820-828.
- Rawat M, Varshney A, Rai M, Chikara A, Pohty AL, Joshi A, et al. (2023). A comprehensive review on nutraceutical potential of underutilized cereals and cereal-based products. Journal of Agriculture and Food Research. 12(1):100619.
- Ayo J, Ayo V, Johnson R. (2020). Chemical composition, physical and sensory qualities of acha-guava flour blend biscuits. Fuoye J Agric Hum Ecol. 3(2):1-12.
- Anyiam PN, Nwuke CP, Uhuo EN, Ije UE, Salvador EM, Mahumbi BM. (2023). Effect of fermentation time on nutritional, antinutritional factors and in-vitro protein digestibility of macrotermes nigeriensis-cassava mahewu. Measurement: Food. 11(2023):100096.
- Marston K, Khouryieh H, Aramouni F. (2016). Effect of heat treatment of sorghum flour on the functional properties of gluten-free bread and cake. LWT-Food Science and Technology. 65:637-644.
- Ayo J, Adedeji O, Okpasu A. (2018). Effect of added moringa seed paste on the quality of acha-moringa flour blends. Asian Food Sci J. 1(2):1-10.
- Aluko RE. (2008). Food protein structures, functionality and product development. Journal of Chemistry. 1(3):22-30.
- AOAC. (2012). Official Methods of Analysis. 19th ed. Gaithersburg (MD): Association of Official Analytical Chemists.
- Girgih AT, Udenigwe CC, Aluko RE. (2011). In vitro antioxidant properties of hemp seed (Cannabis sativa L.) protein hydrolysate fractions. Journal of the American Oil Chemists' Society. 88(3):381-389.
- Owuka GI. (2005). Food analysis and Instrumentation: Theory and Practice. Lagos (Nigeria): Naphthal Prints. pp. 133-137.
- Maiyo NC, Khamis FM, Okoth MW, Abong GO, Subramanian S, Egonyu JP, et al. (2022). Nutritional Quality of Four Novel Porridge Products Blended with Edible Cricket (Scapsipedus icipe) Meal for Food. Foods. 11(7):1047.
- Bolarinwa IF, Olaniyan SA, Adebayo LO, Ademola AA. (2015). Malted sorghum-soy composite flour: Preparation, chemical and physico-chemical properties. J Food Process Technol. 6(8):467.
- Duda A, Adamczak J, Chełmińska P, Juszkiewicz J, Kowalczewski P. (2019). Quality and nutritional/textural properties of durum wheat pasta enriched with cricket powder. Foods. 8(2):46.
- Ghosh A, Ostrander JS, Zanni MT. (2017). Watching proteins wiggle: Mapping structures with two-dimensional infrared spectroscopy. Chem Rev. 117(16):10726-10459.
- González CM, Garzón R, Rosell CM. (2019). Insects as ingredients for bakery goods: A comparison study of Hermetia illucens, Acheta domesticus. A. domestica and T. molitor flours. Innovative Food Science & Emerging Technologies. 51:205-210.
- Olapade AA, Aworh OC. (2012). Evaluation of extruded snacks from blends of acha (Digitaria exilis) and cowpea (Vigna unguiculata) flours. Agric Eng Int: CIGR Journal. 14(3):210-217.
- El Hosry L, Sok N, Richa R, Al Mashtoub L, Cayot P, Bou-Maroun E. (2023). Sample preparation and analytical techniques in the determination of trace elements in food: A review. Foods. 12(4):895.
- Rao SS, Yu S, Fedewa A. (2015). Systematic review: dietary fibre and FODMAP-restricted diet in the management of constipation and irritable bowel syndrome. Aliment Pharmacol Ther. 41(12):1256-1270.
- Mbaeyi-Nwaoha IE, Uchendu NO. (2016). Production and evaluation of breakfast cereals from blends of acha and fermented soybean paste (okara). J Food Sci Technol. 53(1):50-70.
- Marjanovic I, Karan-Djurasevic T, Ugrin M, Virijevic M, Vidovic A, Tomin D, et al. (2017). Use of Wilms Tumor 1 Gene Expression as a Reliable Marker for Prognosis and Minimal Residual Disease Monitoring in Acute Myeloid Leukemia With Normal Karyotype Patients. Clin Lymphoma Myeloma Leuk. 17(5):312-319.
- Bessette AP. (2018). Techno-economic and life cycle assessment of hydrothermal processing of microalgae for biofuels and co-product generation. Old Dominion Univ. DOI: 10.25777/rxmd-g747.
- Madhavan Unny N, Zarina A, Beena V. (2023). Fluid and Electrolyte Balance. In: Textbook of Veterinary Physiology. Springer. pp. 193-211.
- De Loof A, Schoofs L, Huybrechts R. (2016). The endocrine system controlling sexual reproduction in animals: Part of the evolutionary ancient but well conserved immune system? Gen Comp Endocrinol. 226:56-71.
- Zhang ZY, Monleon D, Verhamme P, Staessen JA. (2018). Branched-chain amino acids as critical switches in health and disease. Hypertension. 72(5):1012-1022.
- Godswill AG, Somtochukwu IV, Ikechukwu AO, Kate EC. (2020). Health benefits of micronutrients (vitamins and minerals) and their associated deficiency diseases: A systematic review. Int J Food Sci. 3(1):1-32.
- Morais DR, Rotta EM, Sargi SC, Bonafe EG, Suzuki RM, Souza NE, et al. (2017). Proximate composition, mineral contents and fatty acid composition of the different parts and dried peels of tropical fruits cultivated in Brazil. J Braz Chem Soc. 28(2):308-318.
- Jomova K, Makova M, Alomar SY, Alwasel SH, Nepovimova E, Kuca K, et al. (2022). Essential metals in health and disease. Chem Biol Interact. 367:110173.
- Abraham SI, Ifeanyi N, Stephen S. (2016). Effect of crayfish inclusion on the chemical and sensory properties of ogi prepared from maize, millet and sorghum. Int J Nutr Food Sci. 5(6):378-383.
- Chinwe OU, Ojukwu EO, Jackson BA. (2015). ACHA: A potential grain as a food fortifier. Asian J Agric Food Sci. 3(5):428-435.
- Bessette AP. (2018). Techno-Economic and Life Cycle Assessment of Hydrothermal Processing of Microalgae for Biofuels and Co-Product Generation: Old Dominion University, USA.
- Ibitoye OA, Akinyele BJ, Olaniyi OO. (2019). Evaluation of the nutritional status of Aspergillus flavus-inoculated kati, a cereal-based fermented food. Ann Food Sci Technol. 20(4):776-782.
- Kulamarva AG, Sosle VR, Raghavan GV. (2009). Nutritional and rheological properties of sorghum. International Journal of Food Properties. 12(1):55-69.
- Sarwar MF, Sarwar MH, Sarwar M. (2021). Deficiency of vitamin B-Complex and its relation with body disorders. B-complex vitamins-sources, intakes and novel applications. United Kingdom: IntechOpen. pp. 79-100.
- Ochanda SO, Akoth OC, Mwasaru AM, Kagwiria OJ, Mathooko FM. (2010). Effects of malting and fermentation treatments on group B-vitamins of red sorghum, white sorghum and pearl millets in Kenya. J Appl Biosci. 34:2128-2134.
- Basheer EO, Habib AB, Alhassan IH, Ismaiel AE, Elnour MA, Khalid AM, et al. (2018). Evaluation of microbial aspects and chemical composition of raw beef meat at Khartoum State. Int J Multidiscip Curr Res. 6:1050-1052.
- Banwo K, Oyeyipo A, Mishra L, Sarkar D, Shetty K. (2022). Improving phenolic bioactive-linked functional qualities of traditional cereal-based fermented food (Ogi) of Nigeria using compatible food synergies with underutilized edible plants. NFS Journal. 27:1-12.
- Hrubsa M, Siatka T, Nejmanová I, Vopršalová M, Kujovská Krčmová L, Matoušová K, et al. (2022). Biological properties of vitamins of the B-complex, part 1: Vitamins B1, B2, B3, and B5. Nutrients. 14(3):484.
- Selimovic A, Tissier ML, Arnold W. (2022). Maize monoculture causes niacin deficiency in free-living European brown hares and impairs local population development. Front Ecol Evol. 10:1017691.
- Balwan WK, Kour S. (2022). Thyroid health & methylation: what is the link. Sch J Appl Med Sci. 10(12):2460-2468.
- Thabit JA, Almzaiel AJ. (2023). Emerging Role of Folate-Mediated One Carbon Metabolism in Leukemia: A Review. Egyptian Academic Journal of Biological Sciences. C, Physiology and Molecular Biology. 15(2):215-229.
- Holder KG, Galvan B, Manna PR, Gray ZC, Reddy PH. (2023). Immune enhancers for COVID-19. In: COVID-19 in Alzheimer's Disease and Dementia. p. 49.
- Niki E, Noguchi N. (2021). Antioxidant action of vitamin E in vivo as assessed from its reaction products with multiple biological oxidants. Free Radic Res. 55(4):352-363.
- Aluko RE. (2015). Antihypertensive peptides from food proteins. Annu Rev Food Sci Technol. 6:235-262.
- Sruthi NU, Josna K, Pandiselvam R, Kothakota A, Gavahian M, Mousavi Khaneghah A. (2022). Impacts of cold plasma treatment on physicochemical, functional, bioactive, textural, and sensory attributes of food: A comprehensive review. Food Chem. 368:130809.
- Dumont C, Bourgeois S, Fessi H, Jannin V. (2018). Lipid-based nanosuspensions for oral delivery of peptides: a critical review. Int J Pharm. 541(1-2):117-135.
- Amigo L, Hernández-Ledesma B. (2020). Current Evidence on the Bioavailability of Food Bioactive Peptides. Molecules. 25(19):4479.
- Richardson NE, Konon EN, Schuster HS, Mitchell AT, Boyle C, Rodgers AC, et al. (2021). Lifelong restriction of dietary branched-chain amino acids has sex-specific benefits for frailty and lifespan in mice. Nat Aging. 1(1):73-86.
- Eke-Ejiofor J, Oparaodu F. (2019). Chemical, functional and pasting properties of flour from three millet varieties. Res J Food Nutr. 3(3):15-21.
- Malomo SA, Aluko RE. (2015). A comparative study of the structural and functional properties of isolated hemp seed (Cannabis sativa L.) albumin and globulin fractions. Food Hydrocolloids. 43:743-752.
- Imoisi C, Iyasele J, Michael U, Imhontu E. (2020). The effects of watermelon rind flour on the functional and proximate properties of wheat bread. J Chem Soc Niger. 45(5):978-986.
- Opeifa A, Olatidoye O, Adesala S, Fayomi M. (2015). Production and quality evaluation of ogi produced from fermented maize and horse eye bean (Mucuna urens). Pakistan journal of nutrition. 14(7):417-425.
- Jude-Ojei B, Lola A, Ajayi I, Seun I. (2017). Functional and pasting properties of maize ‘Ogi’supplemented with fermented moringa seeds. J Food Process Techno. 8(5):674.
 Abstract
Abstract  PDF
PDF